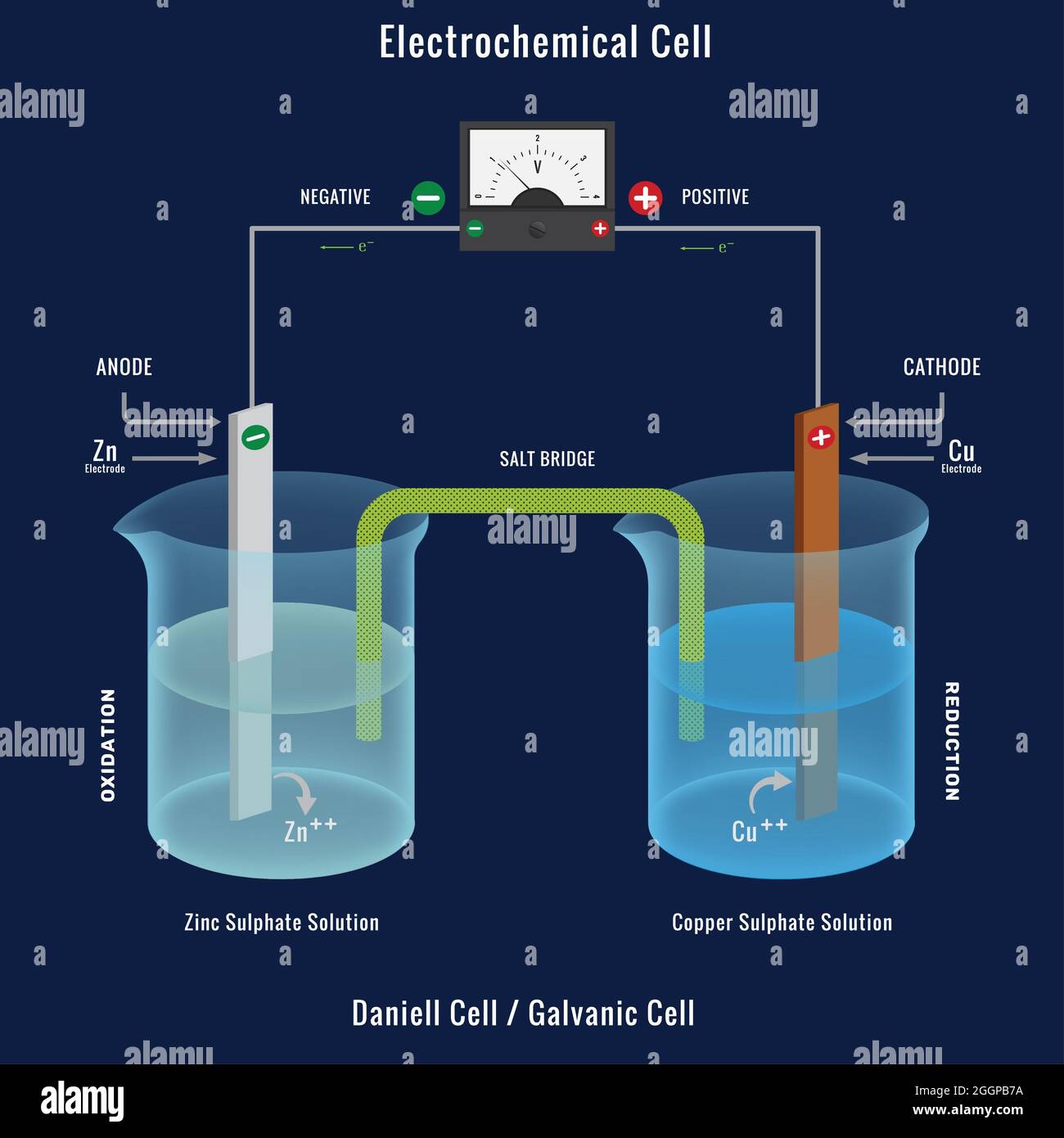
One Example Of Electrochemical Cell . Electrochemistry has many common applications in everyday life. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. This kind of cell includes the galvanic, or voltaic, cell, named after. Reduction (cathode) oxidation (anode) oric acid into hydrogen and.
from www.alamy.com
An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Reduction (cathode) oxidation (anode) oric acid into hydrogen and. Electrochemistry has many common applications in everyday life. This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to.
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter
One Example Of Electrochemical Cell Reduction (cathode) oxidation (anode) oric acid into hydrogen and. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Reduction (cathode) oxidation (anode) oric acid into hydrogen and. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. Electrochemistry has many common applications in everyday life.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter One Example Of Electrochemical Cell All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Electrochemical. One Example Of Electrochemical Cell.
From studylib.net
Electrochemical cells One Example Of Electrochemical Cell Reduction (cathode) oxidation (anode) oric acid into hydrogen and. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell is a device that. One Example Of Electrochemical Cell.
From materialzonesims88.z19.web.core.windows.net
Electrochemical Cells Worksheet One Example Of Electrochemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Reduction (cathode) oxidation (anode) oric acid into hydrogen and. This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemistry has. One Example Of Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME One Example Of Electrochemical Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Reduction (cathode) oxidation (anode) oric acid into hydrogen and. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell splits the oxidant and reductant in a. One Example Of Electrochemical Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii One Example Of Electrochemical Cell All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell is a device that produces an electric current from energy released by a. One Example Of Electrochemical Cell.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses One Example Of Electrochemical Cell This kind of cell includes the galvanic, or voltaic, cell, named after. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemistry has many common applications in everyday life. An apparatus that. One Example Of Electrochemical Cell.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of One Example Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. This kind of cell includes the galvanic, or voltaic, cell, named after. All sorts of batteries, from those used. One Example Of Electrochemical Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells One Example Of Electrochemical Cell All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell. One Example Of Electrochemical Cell.
From lessonschoolbathetic.z5.web.core.windows.net
Electrochemical Cells In Chemistry One Example Of Electrochemical Cell This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell is a device that. One Example Of Electrochemical Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working One Example Of Electrochemical Cell Electrochemistry has many common applications in everyday life. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical. One Example Of Electrochemical Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision One Example Of Electrochemical Cell All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. Electrochemistry has many common applications in everyday life. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell. One Example Of Electrochemical Cell.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples One Example Of Electrochemical Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Reduction (cathode) oxidation (anode). One Example Of Electrochemical Cell.
From www.youtube.com
electrochemical cell electrochemistry class 12 chemistry subject notes One Example Of Electrochemical Cell Reduction (cathode) oxidation (anode) oric acid into hydrogen and. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell splits the oxidant and reductant in a. One Example Of Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells One Example Of Electrochemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. Reduction (cathode) oxidation (anode). One Example Of Electrochemical Cell.
From www.seminarsonly.com
Electrochemical Cell Chemistry Science Fair Project One Example Of Electrochemical Cell All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy.. One Example Of Electrochemical Cell.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram One Example Of Electrochemical Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Reduction (cathode) oxidation (anode) oric acid into hydrogen and. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile,. One Example Of Electrochemical Cell.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID5580086 One Example Of Electrochemical Cell All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Reduction (cathode) oxidation (anode) oric acid into hydrogen and. An apparatus. One Example Of Electrochemical Cell.
From knowledgecycle.in
‘Electrochemical Cell’ Chemistry Investigatory Project PDF » Knowledge One Example Of Electrochemical Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Reduction (cathode) oxidation (anode) oric acid into hydrogen and. Electrochemistry has many common applications in everyday life. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used. One Example Of Electrochemical Cell.